Could it be KC (KERATOCONUS)?
KC File #1: The Patient
Who Corrects to 20/20
Mitch “Private Eye” Ibach OD, FAAO, Vance Thompson Vision

A 29-year-old patient came to our office for a LASIK consult because she was unhappy with fluctuating vision in her contact lenses. The patient had ocular allergies but had no other ocular diagnoses.
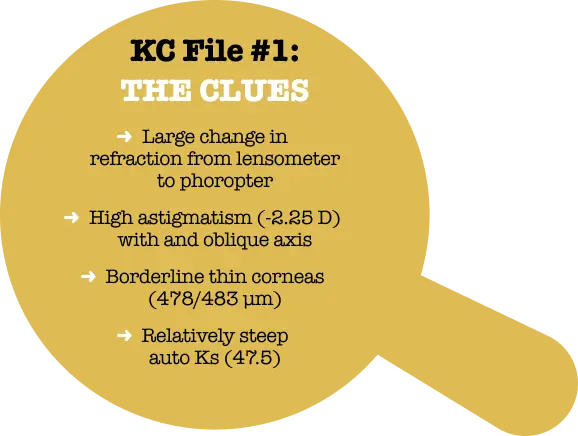
Her entering glasses prescription was a modest one and we were able to refract her to 20/20. However, the refraction in the right eye was our first clue that something was not quite right.
Not only is >2.00 D of refractive cylinder a warning signal for keratoconus, but the oblique axis is also unusual. About 90% of young corneas have with-the-rule (WTR) astigmatism.1 The change in myopic spherical equivalent (SE) from baseline (the glasses prescription) was not what we would expect to see in an adult patient, either.
Refraction and exam findings
| Right Eye | BCVA | Left Eye | BCVA | |
|---|---|---|---|---|
| Lensometry | -0.50 -1.50 x31 | 20/30 | -1.50 -0.50 x172 | 20/20- |
| Refraction at Phoropter | -0.75 -2.25 x34 | 20/20 | -1.75 -0.75 x160 | 20/20+ |
| Pachymetry | 478 μm | 483 μm | ||
| Autokeratometry | 45.5 / 47.50 x 112 | 44.9 / 46.75 x 80 |
REFERENCES
- Kojima T, et al. Am J Ophthalmol 2020;215:127-34,
- Wang Y, et al. Am J Med Genet 2000;93(5):403-9.
- Ferdi AC, et al. Ophthalmology 2019;126(7):935-45.