Could it be KC (KERATOCONUS)?
KC File #2: Autorefractor Clues That Were Ignored
Susan “Super Sleuth” Gromacki, OD, MS, FAAO, FSLS, First Sight Vision Care

A young woman who had relocated to my area for her first job after college was referred to me by the hometown optometrist who had seen her regularly since childhood. She was a high myope, wearing a spectacle prescription of -4.75 sphere OD and -9.25 -1.25 x003 OS.
The optometrist’s records showed a slow but steady decline in best-corrected visual acuity (BCVA). This went unremarked until 2022, when the patient’s OS could not be corrected even to 20/30. At that point, she was referred to a retina specialist, who ruled out a suspected epiretinal membrane and reported other findings all within normal limits.
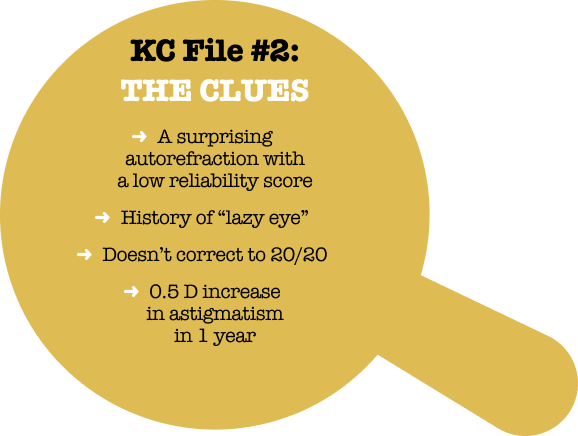
In retrospect, there were three clues that could have alerted the practitioner to the possibility of keratoconus. First, when a young person can’t be corrected to 20/20, the cornea is a more likely culprit than the retina. The patient’s vision in the left eye hadn’t been a sharp 20/20 for several years. Secondly, an increase of 0.5 D of astigmatism between annual visits is a significant red flag. And finally, another clue came from the simplest diagnostic tool in the office-the autorefractor. The autorefraction in 2022 showed 2.75 D of astigmatism-a full diopter more than the spectacle prescription-and the quality score was only 8 out of 10. I would expect it to be 10/10 in a healthy young person.
A history of “lazy eye” as a child may be why the declining BCVA in her left eye was not taken very seriously at first. This was noted in her chart, although there was no evidence of binocular vision testing, no mention of exophoria or esophoria, and she didn’t have the typical hyperopic refraction we usually see in a lazy eye. Sometimes practitioners label a worse-seeing eye as a “lazy eye.” While this certainly may be the case, that label threw off her long-time optometrist. The clues could have led the optometrist to a KC diagnosis.
In 2023, when I first saw this patient, corneal topography showed 2.80 D of astigmatism OS with a classic pattern of inferior steepening that is pathognomonic for progressive KC. She was treated with iLink® corneal cross-linking in the left eye and we continue to follow her right eye closely because KC is a bilateral, asymmetric, disease. Recently the patient shared with me that her uncle has KC, something she didn’t know when I asked her initially.
By following the KC clues that are hiding in plain sight, you can help patients like this one avoid years of declining vision-and sleuth out the right specialists to treat the underlying progressive condition that is stealing her sight. Visit iDetectives.com to learn more.
Changing refractive astigmatism and vision:
| YEAR | DC | BCVA |
| 2014 | 1.00 | 20/20 |
| 2015 | 1.25 | 20/20 |
| 2017 | 1.25 | 20/20-1 |
| 2018 | 1.25 | 20/20 |
| 2019 | 1.25 | 20/25 |
| 2021 | 1.25 | 20/20-2 |
| 2022 | 1.75 | 20/30-1 |
| 2023 | 2.25 | 20/30 |